Products
艾基生物提供高质量的产品以满足客户的需求。 所有产品都经过内部使用和严格的质控, 请大家放心使用
Hot line:
020-89053723


Catlog No:P600-S
Size:16人份/盒
Microsatellite Instability (MSI) Detection Kit Product Manual
(PCR-Capillary Electrophoresis Method)
Microsatellites are short tandem repeat sequences distributed throughout the human genome. Microsatellite instability (MSI) occurs in tumor tissues when there are changes in the length of microsatellites due to insertions or deletions of repeat units compared to normal tissues. MSI is closely associated with defects in mismatch repair (MMR) genes and is significantly linked to tumorigenesis. Clinically, MSI serves as an important molecular marker for prognosis and the development of adjunctive treatment strategies for colorectal cancer, as well as assisting in Lynch syndrome screening.
Product Introduction
This kit utilizes multiplex fluorescent PCR to amplify detection sites, followed by capillary electrophoresis for product detection, and utilizes analysis software for result interpretation. Its main advantages include accurate and comprehensive detection, simple and quick operation, and easy-to-read results.
Detection Sites
Bat26, NR27, Bat25, NR24, Mono27
Product Code
P600-S (16 reactions/kit)
Storage Conditions and Expiry Date
1. Upon receipt of the kit transported on dry ice or ice packs, if not immediately used, store at -20±5°C for long-term preservation.
2. Shelf life: Store at -20°C for 12 months. Upon opening, avoid repeated freeze-thaw cycles. The kit is valid for up to 5 freeze-thaw cycles and remains valid for two months after opening.
Pre-amplification Reagent Kit
| 试剂 | 规格 | 成分 |
| Reaction Mix(2X) | 160μL×1支 | 含PCR缓冲液 |
| Primers Mix | 65μL×1支 | 含检测位点的引物 |
| 热启动酶 | 13μL×1支 | 5 U/μL |
| sdH2O | 500μL×1支 | 超纯水 |
Post-amplification Reagent Kit
| LIZ-500 | 16μL×1支 | 荧光分子量内标 |
PCR thermal cycler
ABI capillary electrophoresis system (e.g., 3500, 3730)
96-well plate centrifuge
Clean bench
Micropipettes
Refrigerator
Protocol for Using the Reagent Kit (Please gently shake and briefly centrifuge the reagents before use):
Preparation of Genomic DNA
Use a genomic DNA extraction kit to extract genomic DNA from the samples to be tested. For example, the iPure Cell/Blood/Animal Tissue gDNA Extraction Kit (K316-L) from Guangzhou Aijie Biological Technology Co., Ltd.
PCR Amplification
PCR Reaction System (PCR reaction mix is prepared immediately before use, any excess mix can be stored at -20°C for up to one week, avoid repeated freeze-thaw cycles.)
| 基因组 DNA | 30ng-100ng 基因组 DNA |
| Reaction Mix (2X) | 10.0μL |
| Primers Mix | 3.0μL |
| 热启动C-Taq酶 | 0.8μL |
| sdH2O | 补水至20.0μL |
2.2 PCR Program:
| 步骤 | 温度 | 时间 | 循环数 |
预变 | 95℃ | 5mi | |
热循环 | 95℃ | 30s | 30 |
56℃ | 90s | ||
70℃ | 30s | ||
终延伸 | 70℃ | 30min | |
保温 | 15℃ | ∞ |
Capillary Electrophoresis Detection:
After centrifuging the PCR amplification product at 3000rpm for 1 minute, take 1μL of the PCR amplification product and mix it with 1μL of LIZ-500 and 9μL of deionized formamide (deionized formamide needs to be prepared by yourself). After centrifugation of the 96-well plate, perform capillary electrophoresis detection.
Data Analysis:
After importing the panel and bin files, analyze the raw data using GeneMapper, and judge the results according to the microsatellite instability evaluation criteria. (Please contact the company for the Panel and bin files).
Evaluation Criteria:
(1) High Instability (MSI-H): 2 or more mutations appear in 5 molecular markers.
(2) Low Instability (MSI-L): Only 1 mutation appears in 5 molecular markers.
(3) Microsatellite Stable (MSS): No mutation appears in 5 molecular markers.
Peak Chart Example:
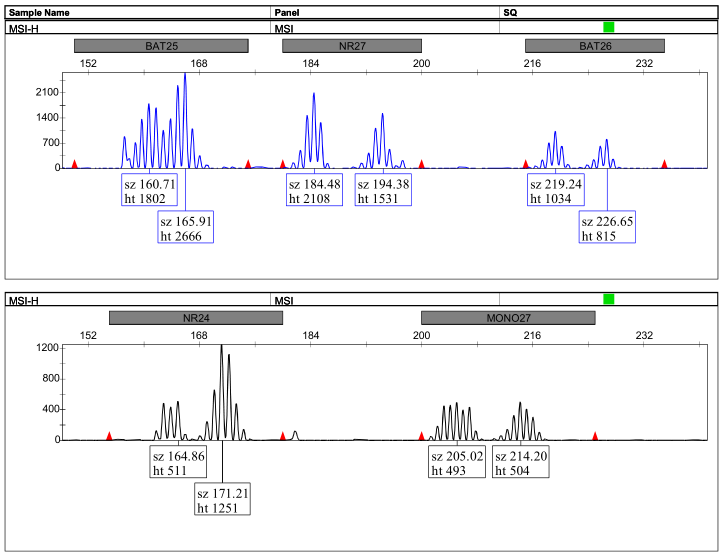
图1:高度不稳定(MSI-H)

图2:微卫星稳定(MSS)
Sample Requirements:
Sample Type: Human EDTA anticoagulated whole blood.
Sample Storage: Blood samples should be sent for testing as soon as possible after collection; or stored at -20°C ±5°C for up to 24 months. Once genomic DNA is extracted, it should be tested as soon as possible; or stored at 2~8°C for up to 4 weeks; or stored at -20°C ±5°C for up to 24 months, with no more than 5 freeze-thaw cycles.
Sample DNA Quality Requirements: DNA should ensure an OD260/OD280 ratio between 1.6 and 2.0, with a concentration ≥10 ng/μL.
Precautions:
Please read the product manual carefully before use.
The laboratory should have reasonable biosafety facilities and protection procedures. Laboratory management should strictly follow the management specifications of PCR laboratories, and experimental operators should have qualifications related to gene amplification and other relevant experimental operations. PCR-related operations should be strictly conducted in accordance with relevant national regulations for strict zoning. Special instruments and equipment should be used for each stage of the experimental operation, and supplies from different areas and stages should not be cross-used to prevent nucleic acid cross-contamination.
Centrifuge tubes and tips used in experiments should be sterile and free of nucleases.
During the experiment, wear protective clothing, disposable gloves, and masks.
Residual samples, PCR amplification products, contaminated reagents, and consumables during the testing process should be disinfected and handled in accordance with relevant regulations and laws.
Do not mix reagents from different batches when using the kit within the expiration date.
Enzyme mix-related operations should be performed on ice. Other reagents should be completely thawed and mixed evenly before use, and repeated freeze-thaw cycles should be avoided as much as possible.
When adding samples, all liquids should be slowly added to the bottom of the tube, not left on the tube wall, and the PCR tube lid should not be touched directly with hands.
If the sample has quality issues, such as severe degradation or containing PCR inhibitors, the test results may show spurious peaks or loss of target amplification products, affecting result interpretation.
References:
[1] Br J Cancer. 1998;77:2343-48.
[2] Cancer Res. 1998 Nov 15;58(22):5248-57.
[3] Yonsei Med J. 2009;50(3):309-21.
[4] J Clin Oncol. 2010 Jul 10;28(20):3219-26.
[5] World J Gastrointest Oncol. 2013 Feb 15;5(2):12-19.
[6] J Clin Oncol. 2015 Jan 10;33(2):209-17.
[7] N Engl J Med 2015 June 25;372:2509-20.
[8] Chin J Dig Surg. 2015 Oct;14(10):783-99.
[9] China Cancer. 2015;24(1):1-10.
[10] "NCCN Guidelines colon Cancer Version 3.2017".
[11] "Chinese Guidelines for Diagnosis and Treatment of Colorectal Cancer 2015 Edition".

 Copyright © Guangzhou IGE Biotechnology Co., Ltd.
粤ICP备2021146096号
Copyright © Guangzhou IGE Biotechnology Co., Ltd.
粤ICP备2021146096号


