The detection of circulating tumor DNA (ctDNA) gene mutations has certain clinical value for targeted tumor therapy, early treatment response evaluation, and resistance monitoring. Due to the limitations of tissue samples, clinical practice has gradually shifted towards using ctDNA from patient plasma for tumor gene mutation detection.
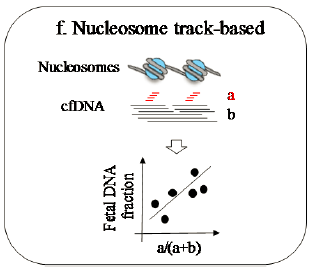
At the same time, a rapid, simple, accurate, and economical method for estimating cfDNA concentration is essential for non-invasive prenatal testing (NIPT), especially considering the future direction of clinical applications for non-invasive single-gene diseases. cfDNA concentration can affect the accuracy of NIPT results and also holds potential clinical diagnostic value.
This reagent kit is designed based on the characteristics of nucleosome fragmentation, with specific primers and probes. The kit contains pre-mixed master mix (hotstart taq polymerase, dNTPs, probe, primer, buffer), negative control, positive control, sample dilution solution, and other components, facilitating customer operation. It is used for internal quality control of circulating DNA. This kit can only determine whether the correct ctDNA is extracted from the sample and cannot quantify it.
Product Features:
Simple Operation: Pre-mixed master mix, no need for any other reagents, simple operation, fixed amplification program, reliable data. It has a lower detection limit than picogreen detection, as low as 50 copies/μl.
Widely Applicable Platform: Suitable for detection on any fluorescence quantification platform, widely applicable to instruments such as ABI7500, 7500fast, StepOne, StepOne Fast, Applied Biosystems 3000XP, Roche LightCycler 480, Shanghai Hongshi, Hangzhou Borui, Xi'an Tianlong, etc.
Stable Data: High repeatability, using specific probe sequences greatly improves the specificity lacking in traditional SYBR green dye methods, with inter-batch repeatability CV < 5%.


