The circulating DNA (Cell-free DNA or cfDNA) is extracellular DNA present in bodily fluids such as plasma, serum, amniotic fluid, cerebrospinal fluid, etc. It is a degraded endogenous DNA & RNA circulating freely outside the cells.
As early as 1947, Mandel and Metais discovered circulating free nucleic acids, but due to the lack of highly sensitive and specific experimental methods, research on the relevance of circulating DNA in blood to diseases progressed slowly for a long time.
In 1966, Dr. Henry H Kunkel and colleagues at Rockefeller University in New York reported in the prestigious medical journal J Clin Invest that circulating DNA was present in the serum of some systemic lupus erythematosus patients. This DNA could react with certain antibodies in the serum, thereby participating in the pathological process of the disease.
In 1987, Dr. Philippe Anker and Maurice Stroun at the University of Geneva in Switzerland discovered the presence of circulating DNA in the peripheral blood of cancer patients.
In 1997, YMD Lo and others reported in the internationally renowned medical journal Lancet that Y chromosomes were detected in the peripheral blood of pregnant women carrying male fetuses, proving the presence of fetal circulating DNA in the peripheral blood of pregnant women. Subsequent studies found that the detection time of fetal cfDNA in maternal peripheral blood is mainly related to gestational age, with earlier gestational ages corresponding to lower fetal components. This discovery is of great diagnostic significance for non-invasive prenatal testing of pregnant women. With the development of biotechnology and medical technology, the detection of circulating DNA and the study of its biological indicators will provide a series of convenient, rapid, specific, and non-invasive technical means for genetic diseases, early diagnosis of tumors, postoperative testing, follow-up, prenatal non-invasive diagnosis, etc.
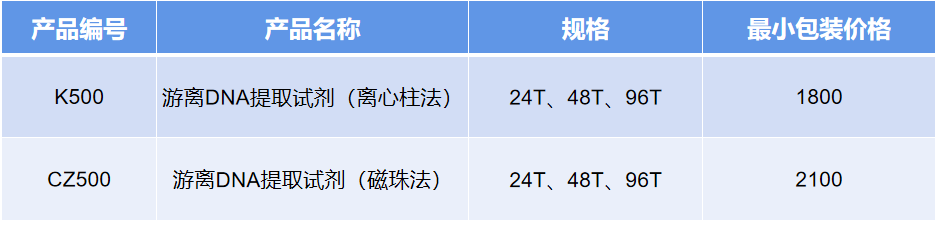
Main supporting application projects:
Gene mutation detection in tumors (non-small cell lung cancer, breast cancer, colon cancer, cervical cancer, etc.).
Gene mutation detection in systemic genetic diseases (systemic lupus erythematosus, polydactyly, congenital deaf-muteness, hemophilia, etc.).
Non-invasive DNA prenatal testing (Y chromosome abnormalities, trisomy 21 syndrome, trisomy 13 syndrome, trisomy 18 syndrome, etc.).

Plasma cfDNA Preparation System Optimized for Non-Invasive Diagnostics
The highly dispersed superparamagnetic silica nanoparticles have good adsorption specificity and can efficiently and specifically bind to DNA fragments. The plasma DNA preparation system developed based on the principle of magnetic bead adsorption is characterized by simple and fast operation and does not require toxic organic solvents. The prepared DNA can be directly applied to library construction.
Advantages:
Fast, automatic extraction of 20 samples by one person within 35 minutes.
Up to 4mL of plasma can be extracted at a time, ensuring that the DNA quantity meets the requirements for subsequent sequencing.
High DNA extraction efficiency, with an average DNA yield of 15ng from 2mL of plasma, sufficient for library construction and downstream applications such as qRT-PCR and enzyme-linked reactions.
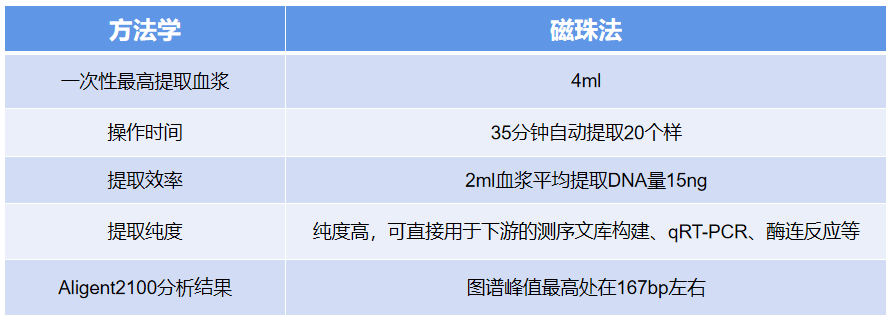
Clinical stage III lung cancer plasma samples were purified using the iPure Cell-Free DNA kit and Company A's product (Cat.NO. A29319), and the purified DNA was analyzed for DNA fragment range using the Aligent 2200. The results showed that iPure's iPure Cell-Free DNA kit had a higher recovery and extraction efficiency for small 160bp DNA fragments.

Guide