Service introduction
Adenovirus (Ad) is one of the most efficient and reliable recombinant virus expression systems available. It can be used to transiently and efficiently express target genes in mammalian cells. It has the following advantages:
Wide host range and high infection efficiency
Effective replication and high virus titer
No integration into the host genome and high biosafety
IGEBio uses the AdMAX™ system to construct recombinant adenoviruses. First, the adenovirus vector is produced by the method of in vitro recombination of shuttle plasmids and backbone plasmids. Then, it is further packaged and amplified in 293A cells to obtain high-titer recombinant Ad.
Service advantages
Professional vector construction services: A variety of shuttle vectors to meet different customer needs, relying on our own molecular cloning platform, more professional services.
Standard adenovirus packaging process: High virus titer, short cycle, good safety.
One-stop service: From vector design, construction, synthesis, sequencing identification to virus packaging, concentration and titer determination, all are completed by our professional team.
Services provided
Conventional gene overexpression/interference adenovirus packaging
CircRNA/LncRNA overexpression and interference adenovirus packaging
Adenovirus in stock
Technical procedures
IGEBio adenovirus system composition
An expression vector containing the target gene, the promoter of the target gene, and some elements that package this vector into the virus.
A helper plasmid.
293A cell line, which can package adenovirus after transfection with the expression vector.
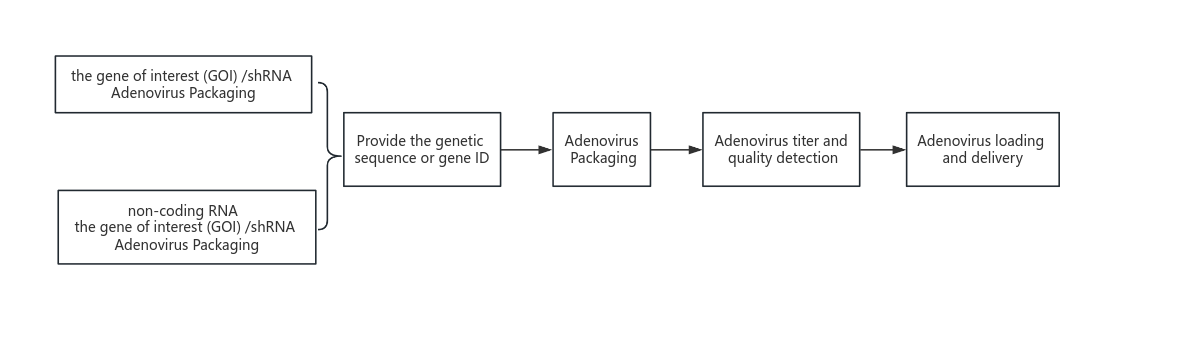
Customized virus products
Note:
This cycle does not include vector construction. The overall experimental plan, quotation and cycle will be given after experimental analysis.
If the customer provides an adenovirus vector, the corresponding plasmid map needs to be provided for quality inspection of the plasmid. Please send the vector to us strictly in accordance with our plasmid requirements. All project materials need to be inspected by QC. Each material will require additional inspection fees. The project will officially start after the customer provides the items and passes QC.
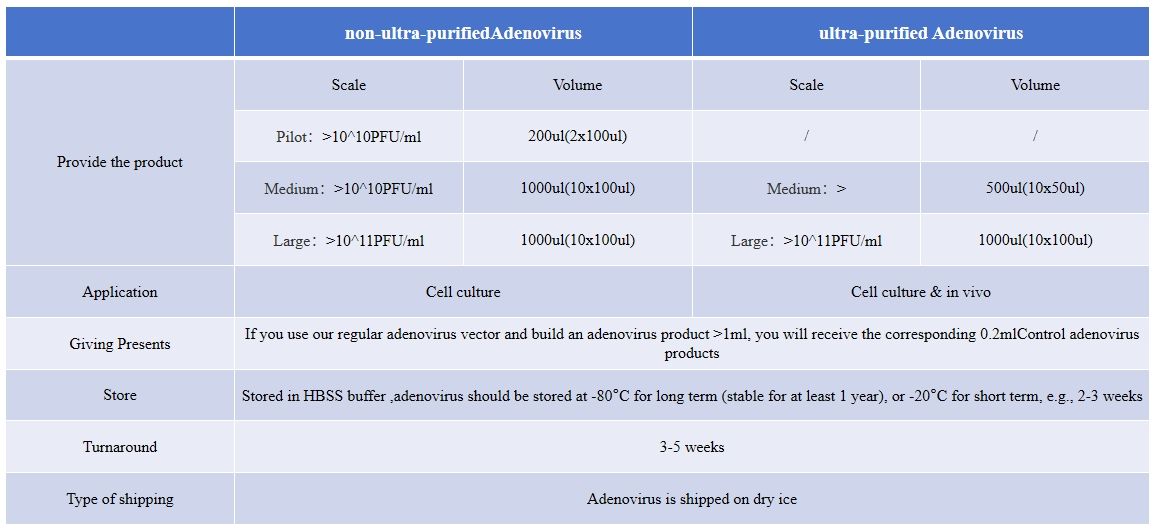
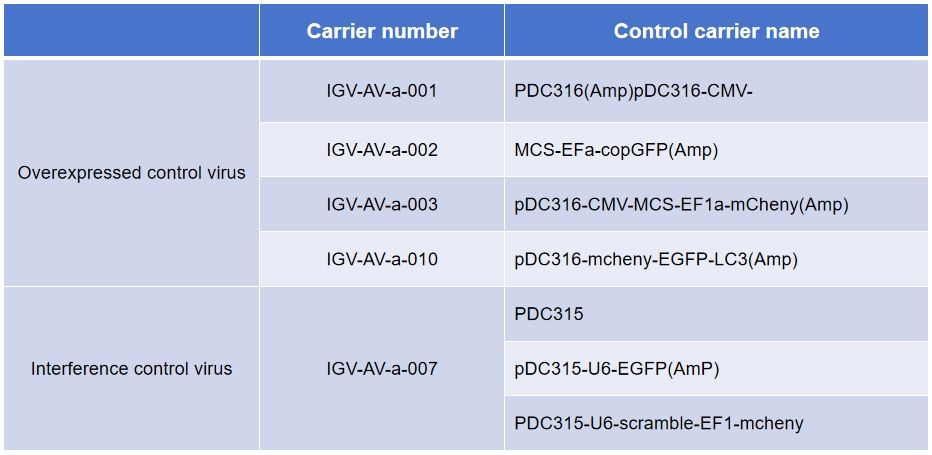
Control virus products
Note:
The delivery cycle of the control virus is 1 week after the contract is signed.
Adenovirus is stored in HBSS buffer and transported by dry ice.
Virus product quality assurance
Titer determination: The titer of adenovirus is determined by the TCID50 method. The adenovirus is diluted by a gradient, then infected with 293A cells, and 10 wells are inoculated for each gradient. After 8-10 days, the CPE phenomenon of each well is observed. The wells with CPE are positive, and the wells without CPE are negative. The results are calculated according to the Reed-Muench two-person method.
Note:
The transfection efficiency of the same type of cells under different transfection conditions can be 2-5 times different for the same virus liquid. The difference may be even greater between different types of cells.
If the qpcr titer verification results are required, please indicate in advance. Generally, the delivery does not include qpcr test data.
2. Sterility test: The culture medium inoculated with virus storage liquid has been tested for the growth of bacteria and fungi.
Case presentation
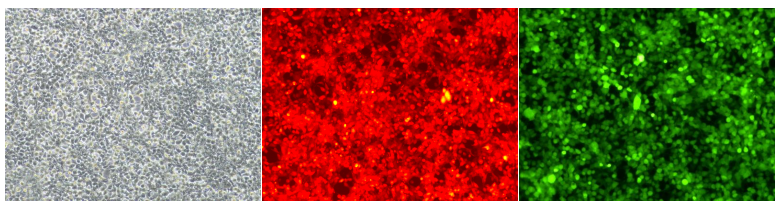
Fig1. The effect of 293T cells being infected under the condition of MOI=1

